X Chromosome Inactivation: Breakthrough in Genetic Research
X chromosome inactivation plays a crucial role in cellular biology, particularly in female individuals who possess two X chromosomes. Unlike males with a single X chromosome, females must inactivate one of these chromosomes to ensure gene dosage balance. This intricate process has significant implications for various genetic disorders, including Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome lead to profound developmental challenges. Researchers like Jeannie Lee have uncovered fascinating insights into chromosomal silencing mechanisms, revealing how a gel-like substance assists in this inactivation process. Such discoveries could lead to groundbreaking therapeutic strategies for millions affected by disorders tied to the X chromosome.
The phenomenon of X chromosome dosage compensation is vital for female cells, which face the unique challenge of having two X chromosomes. To manage this genetic complexity, one X is silenced, a process intricately linked to various chromosomal behaviors and gene expression management. This process, often referred to as chromosomal silencing, directly influences the understanding of conditions like Fragile X Syndrome and Rett Syndrome. Jeannie Lee’s pioneering research sheds light on how certain substances interact with the X chromosome, providing new avenues for addressing these genetic disorders. The potential to reactivate dormant genes offers hope for therapeutic advancements that could transform lives.
Understanding X Chromosome Inactivation
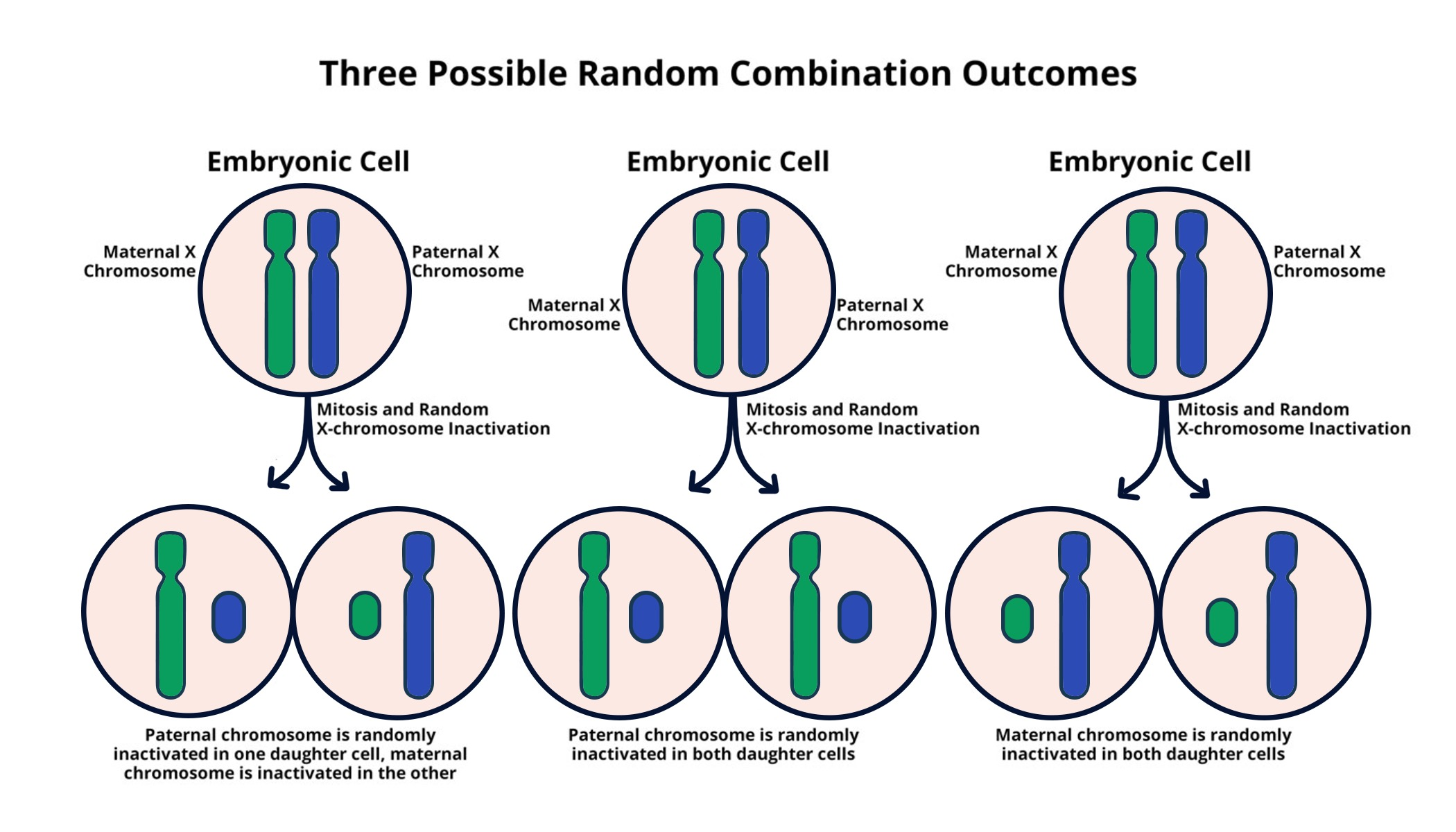
X chromosome inactivation (XCI) is a crucial biological process that ensures dosage compensation between males and females. In females, with two X chromosomes, one X is randomly inactivated in each cell early in development, allowing dosage balance of X-linked genes with males, who possess only one X chromosome. This unique process requires complex mechanisms, where genes such as Xist play a vital role. Xist produces an RNA molecule that coats and silences one of the X chromosomes, effectively shutting down its expression and creating an equilibrium between the sexes.
The significance of X chromosome inactivation extends beyond mere genetic balance. It has profound implications for various genetic disorders, including Fragile X Syndrome and Rett Syndrome, both of which are associated with mutations on the X chromosome. Understanding how XCI operates, as detailed by Jeannie Lee’s research, opens pathways to potential therapeutic interventions aimed at these conditions. Through better comprehension of the silencing mechanisms, researchers hope to develop innovative treatments that can reactivate the inactivated X chromosome carrying the healthy gene.
Implications of Chromosomal Silencing in Genetic Disorders
Jeannie Lee’s groundbreaking research highlights the mechanics of chromosomal silencing and its implications for genetic disorders caused by mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. By revealing how Xist interacts with the ‘Jell-O’ like substance surrounding chromosomes, Lee’s lab provides insights into the fundamental processes that lead to gene silencing. This understanding could be pivotal in devising strategies to treat individuals affected by these disorders, where therapeutic treatments could target the reversing of X chromosome inactivation, allowing reexpression of vital genes.
The concept of chromosomal silencing not only applies to females but extends to males, particularly when dealing with specific mutations that silence individual X-linked genes. The potential for treating Fragile X and Rett Syndromes is exhilarating; as research progresses, the focus is on developing compounds to unsilence these genes. If successful, such approaches could restore normal function to previously silenced genes with minimal side effects, as observed in ongoing studies. This presents a promising avenue for therapeutic strategies aimed at alleviating symptoms of these debilitating genetic disorders.
Exploring Treatment Avenues for Fragile X and Rett Syndromes
The ongoing studies led by Jeannie Lee’s team are focused on the restoration of function to mutated genes associated with Fragile X Syndrome and Rett Syndrome. By developing techniques to unsilence inactivated X chromosomes, researchers are paving the way for innovative treatments that could fundamentally change the management of these conditions. Fragile X Syndrome, which leads to intellectual disabilities and developmental challenges, could potentially see benefits from advancements in gene therapy targeting XCI mechanisms.
Similarly, Rett Syndrome, characterized by severe neurodevelopmental impairment, may benefit from therapies focused on the reactivation of genes lost due to X chromosome silencing. The approach taken involves ensuring that while the pathological gene is addressed, the healthy gene remains unaffected. This delicate balance is crucial for minimizing side effects. Therefore, pharma collaborations and clinical trials are expected as these research efforts transition from the laboratory to practical treatment solutions for affected individuals.
The Role of Jeannie Lee and Her Research
Jeannie Lee has been at the forefront of X chromosome research, providing significant insights into the mechanisms underlying X chromosome inactivation. Her extensive research, bolstered by 25 years of funding from the National Institutes of Health, culminated in a breakthrough that holds therapeutic promise for genetic disorders. Lee’s lab is dedicated to unraveling the complexities of gene silencing and its repercussions on diseases like Fragile X and Rett syndromes, striving towards findings that can be clinically applied.
Lee’s passion for genetic research stems from the potential real-world applications that may arise from her scientific discoveries. The emerging understanding of chromosomal silencing enhances the ability to devise targeted therapies to reactivate silenced genes, providing hope for those living with X-linked disorders. As the scientific community watches her progress, the anticipation builds for tangible advancements that could dramatically improve countless lives influenced by these genetic challenges.
Mechanics of X Chromosome Silencing in Depth
The mechanics behind X chromosome silencing, primarily facilitated by Xist, showcase an intricate process essential for proper gene expression regulation. When Xist RNA binds to the X chromosome, it alters the structure and biochemical properties of the surrounding chromatin, leading to a state of transcriptional inactivity. This process is not only revolutionary for understanding female-specific gene regulation but also offers insights into potential therapeutic targets for silencing diseases, particularly those attributed to mutations on the X chromosome.
An intriguing aspect of this process is the biophysical interaction between Xist and the ‘Jell-O’ substance. As Xist penetrates this gel-like environment, it modifies its properties, paving the way for other molecules to engage and contribute to the inactivation. The revelations from Lee’s research provide a blueprint for exploring how to manipulate these interactions to unsilence genes, thereby presenting groundbreaking implications for treating genetic disorders caused by X-linked mutations.
Future Directions in X Inactivation Research
The future objectives within Jeannie Lee’s laboratory involve taking the findings related to X chromosome inactivation to the next level, with a focus on clinical applications. This includes optimizing the techniques developed for unsilencing X-linked genes to ensure safety and efficacy before moving into clinical trials. The journey from basic research to therapeutic application is critical and reflects a significant shift towards precision medicine in treating genetic disorders such as Fragile X Syndrome and Rett Syndrome.
Moreover, ongoing studies are aimed at understanding not just the mechanics of XCI, but why certain mutations lead to the silencing of pathological genes while leaving healthy genes intact. If successful, unraveling these mysteries could lead to highly refined and targeted interventions that not only offer treatment but also improve the quality of life for patients. This research represents a promising frontier in genetics and biomedicine, with the potential to change how genetic disorders are approached comprehensively.
The Significance of Genetic Research Funding
Research funding has been a critical element in advancing genetic discoveries, particularly in understanding X chromosome inactivation. Jeannie Lee’s work, supported by the National Institutes of Health, highlights the importance of sustained financial backing for cutting-edge scientific exploration. Over the years, investment in fundamental research has led to breakthroughs that may ultimately inform regulatory approvals for innovative approaches to gene therapy targeting disorders like Fragile X and Rett syndromes.
The continuity of such funding allows researchers to remain dedicated to unraveling complex biological processes essential for treating genetic conditions. It enables the employment of advanced technologies and methodologies that can drive discoveries from the lab to clinical settings. Therefore, advocacy for research funding is paramount to ensure that vital questions in genetic disorders are addressed, paving the way for tomorrow’s medical breakthroughs.
Potential Therapeutic Approaches Based on XCI Research
The potential therapeutic strategies emerging from research on X chromosome inactivation are particularly exciting in the context of genetic disorders. Approaches aiming to awaken silenced X-linked genes could result in groundbreaking treatments, especially for conditions stemming from mutations on the X chromosome. As Lee’s lab progresses, examining how to target and reverse the consequences of XCI presents a unique opportunity to address genetic disorders that have long remained challenging in the field of medicine.
In addition, eluding the challenge of selectively targeting activated genes while preserving healthy ones offers a pathway to achieving effective treatments with fewer side effects. This focus on precision raises the prospects of individualized medicine, where therapies can be tailored according to the genetic profile of the patient. As new methods are developed, the application of Lee’s findings could revolutionize the treatment landscape for Fragile X Syndrome, Rett Syndrome, and similar genetic diseases.
Broader Implications of X Chromosome Research
The broader implications of research on X chromosome inactivation extend well beyond individual conditions. Understanding the mechanisms of chromosomal silencing can reshape our knowledge of gene regulation across a spectrum of genetic disorders. Unraveling these complexities not only informs us about maternal inheritance patterns but also about male-specific mutation outcomes, demonstrating the far-reaching influence of genetic studies.
By illuminating how genes can be selectively silenced or reactivated, this area of research may eventually lead to novel diagnostic tools or treatment strategies applicable to a variety of genetic disorders. As Jeannie Lee and her team continue to push the boundaries of what we know about the X chromosome, the insights gained may unlock new frontiers in genetic research and therapy, making profound changes in how we conceptualize and tackle inherited disorders.
Frequently Asked Questions
What is X chromosome inactivation and why is it important?
X chromosome inactivation is a biological process that occurs in female mammals, where one of the two X chromosomes is silenced to ensure equal gene dosage with males, who have only one X chromosome. This process is crucial for preventing gene dosage imbalances that could lead to developmental issues and genetic disorders.
How does X chromosome inactivation relate to genetic disorders like Fragile X Syndrome and Rett Syndrome?
X chromosome inactivation plays a significant role in genetic disorders such as Fragile X Syndrome and Rett Syndrome because these conditions are often linked to mutations on the X chromosome. Understanding how X inactivation works may help develop therapies that could reactivate the unaffected genes on the inactive X chromosome, potentially alleviating symptoms of these disorders.
Who is Jeannie Lee and what is her contribution to the study of X chromosome inactivation?
Jeannie Lee is a prominent researcher at Harvard Medical School who has made significant contributions to understanding X chromosome inactivation. Her lab’s research focuses on the mechanisms involved in chromosomal silencing, which could have implications for treating genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
What are the potential therapeutic implications of unraveling the mechanics of X chromosome inactivation?
Unraveling the mechanics of X chromosome inactivation could lead to new therapies for genetic disorders by enabling scientists to ‘unsilence’ inactivated X chromosomes, thereby allowing the expression of healthy genes that have been silenced due to mutations. This could potentially provide a cure for conditions like Fragile X Syndrome and Rett Syndrome, with fewer side effects.
What role does the RNA molecule Xist play in X chromosome inactivation?
Xist is a pivotal RNA molecule involved in X chromosome inactivation. It is produced by a gene on the X chromosome and interacts with the surrounding jelly-like substance, modifying its properties to facilitate the silencing of the X chromosome. The presence of Xist is essential for the successful chromosomal silencing that characterizes X inactivation.
Can the techniques used in Jeannie Lee’s lab for X chromosome activation be applied to male genetic disorders?
Yes, the techniques developed in Jeannie Lee’s lab for X chromosome activation could also benefit affected males. Though males do not undergo X chromosome inactivation, similar silencing mechanisms can occur in individuals with certain mutations on the X chromosome, which suggests that they could also respond to approaches aimed at reactivating the healthy gene.
What is chromosomal silencing and how does it relate to X chromosome inactivation?
Chromosomal silencing is the process by which specific genes on a chromosome are turned off or rendered inactive. X chromosome inactivation is a specific form of chromosomal silencing that occurs on one of the X chromosomes in females, preventing overexpression of X-linked genes and ensuring equal gene expression levels between sexes.
How do the findings of Jeannie Lee’s lab influence future research and treatment of X-linked genetic disorders?
The findings from Jeannie Lee’s lab provide a foundational understanding of X chromosome inactivation, which influences future research and potential treatments by opening new avenues for reactivating silenced genes. This could lead to more effective therapies for X-linked genetic disorders such as Fragile X Syndrome and Rett Syndrome, improving outcomes for affected individuals.
| Key Point | Detail |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes but only one is active due to inactivation. |
| Gene Xist | The X chromosome produces an RNA molecule called Xist that initiates the inactivation process. |
| Role of ‘Jell-O’ | A jelly-like substance around chromosomes aids in making the X chromosome inactive by allowing Xist to modify it. |
| Potential Therapies | Unlocking inactivated X chromosomes could lead to treatments for disorders like Fragile X and Rett Syndromes. |
| Clinical Trials | Research shows promise for new therapies; optimization and safety studies are planned. |
| Future Research | Further studies may help clarify how to awaken muted genes without affecting healthy ones. |
Summary
X chromosome inactivation is a crucial biological process that allows female cells to effectively manage the presence of two X chromosomes by silencing one. This remarkable mechanism has been extensively studied in Jeannie T. Lee’s lab, shedding light on how chromosomal silencing occurs and paving the way for potential therapeutic applications. With the ability to potentially reactivate inactivated X chromosomes, researchers are optimistic that new treatments for genetic disorders could emerge, especially for conditions linked to X chromosome mutations.